‘It’s the sovereignty of the country’: Guinea-Bissau says US vaccine study suspended
The Guardian • 1/23/2026 – 1/30/2026

Summary
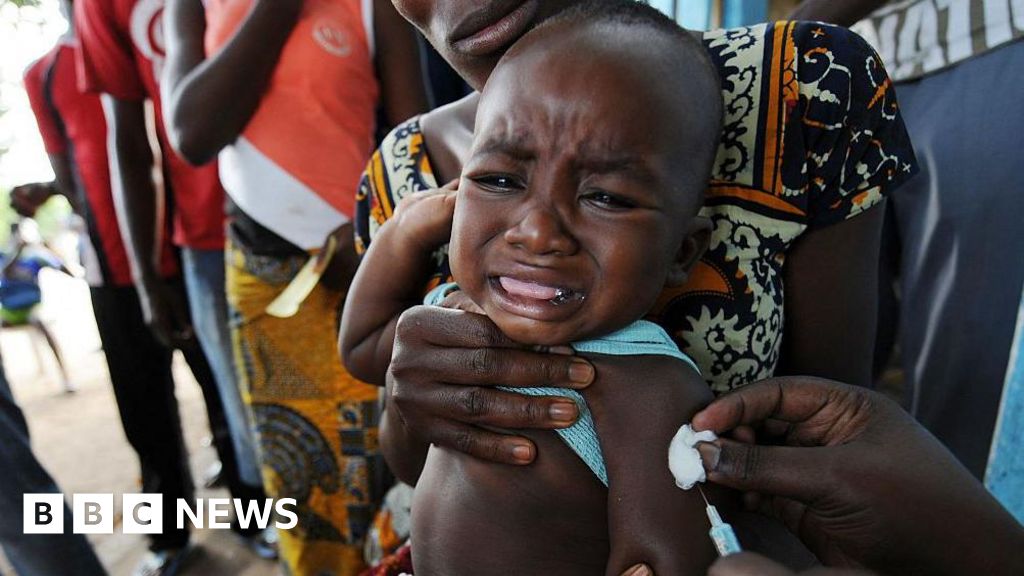
Guinea-Bissau, a West African nation, has announced the suspension of a controversial hepatitis B vaccine study funded by the United States, despite conflicting statements from US health officials who claim the study is still ongoing. The research, which was set to be conducted by Danish scientists, has raised significant ethical concerns, particularly in light of recent changes to the US vaccination schedule. This situation highlights the complexities of conducting medical research in low-income countries. The controversy surrounding this vaccine study underscores broader issues related to global health ethics and the dynamics of international research. It reflects a historical pattern where wealthier nations often impose their health agendas on poorer countries, raising questions about sovereignty and informed consent. The situation in Guinea-Bissau serves as a reminder of the delicate balance between advancing medical knowledge and respecting the rights and autonomy of vulnerable populations. As discussions about ethical research practices continue, this case may influence future policies regarding international health studies, emphasizing the need for transparency and collaboration in global health initiatives.
Advertisement
Lindy Score Breakdown (V4.2)
Score BreakdownRisk 35
Stories gain Lindy status through source reputation, network consensus, and time survival.
Breaking Similar stories








Anti-Lindy Similar stories







