STAT+: Hims is in the crosshairs. Is a crackdown coming for compounding?
Stat News • 2/5/2026 – 2/11/2026
Summary
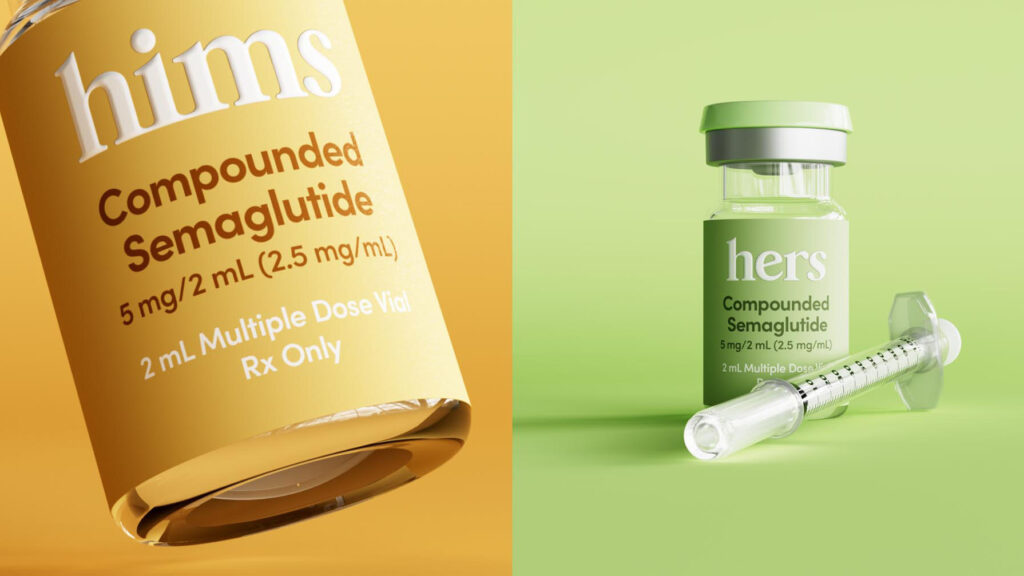
Hims & Hers, a telehealth company, is facing scrutiny from federal regulators regarding its sale of a compounded version of the obesity drug Wegovy. The U.S. Department of Health and Human Services (HHS) has requested the Justice Department to investigate Hims & Hers for marketing this cheaper compounded version, which has drawn criticism from Novo Nordisk, the manufacturer of Wegovy. Novo Nordisk has labeled the product an "untested knockoff," raising concerns about its safety and efficacy. In response to the regulatory pressure, Hims & Hers has announced that it will cease selling the compounded version of Novo Nordisk's obesity pill on its telehealth platform. This decision comes amid a broader federal regulatory crackdown aimed at companies that mass market unapproved compounded versions of GLP-1 drugs, which are used for weight management. The Food and Drug Administration (FDA) has indicated that it will take "decisive steps" to address this issue. The implications of this crackdown could extend beyond Hims & Hers, potentially affecting a wider network of compounding pharmacies and telehealth companies involved in similar practices. The situation highlights the ongoing tensions between regulatory bodies and companies offering alternative pharmaceutical solutions, particularly in the rapidly evolving landscape of telehealth and compounded medications.
Advertisement
Cluster Activity
Lindy Score Breakdown (V4.2)
Story Timeline
- 2026-02-05
- 2026-02-06
- 2026-02-07
- 2026-02-09
- 2026-02-11STAT+: Hims is in the crosshairs. Is a crackdown coming for compounding? (current)
Score BreakdownRisk 35
Stories gain Lindy status through source reputation, network consensus, and time survival.
Same Story from 10 sources






Breaking Similar stories




