STAT+: Investors cheer Compass’ psilocybin data
Stat News • 2/10/2026 – 2/18/2026
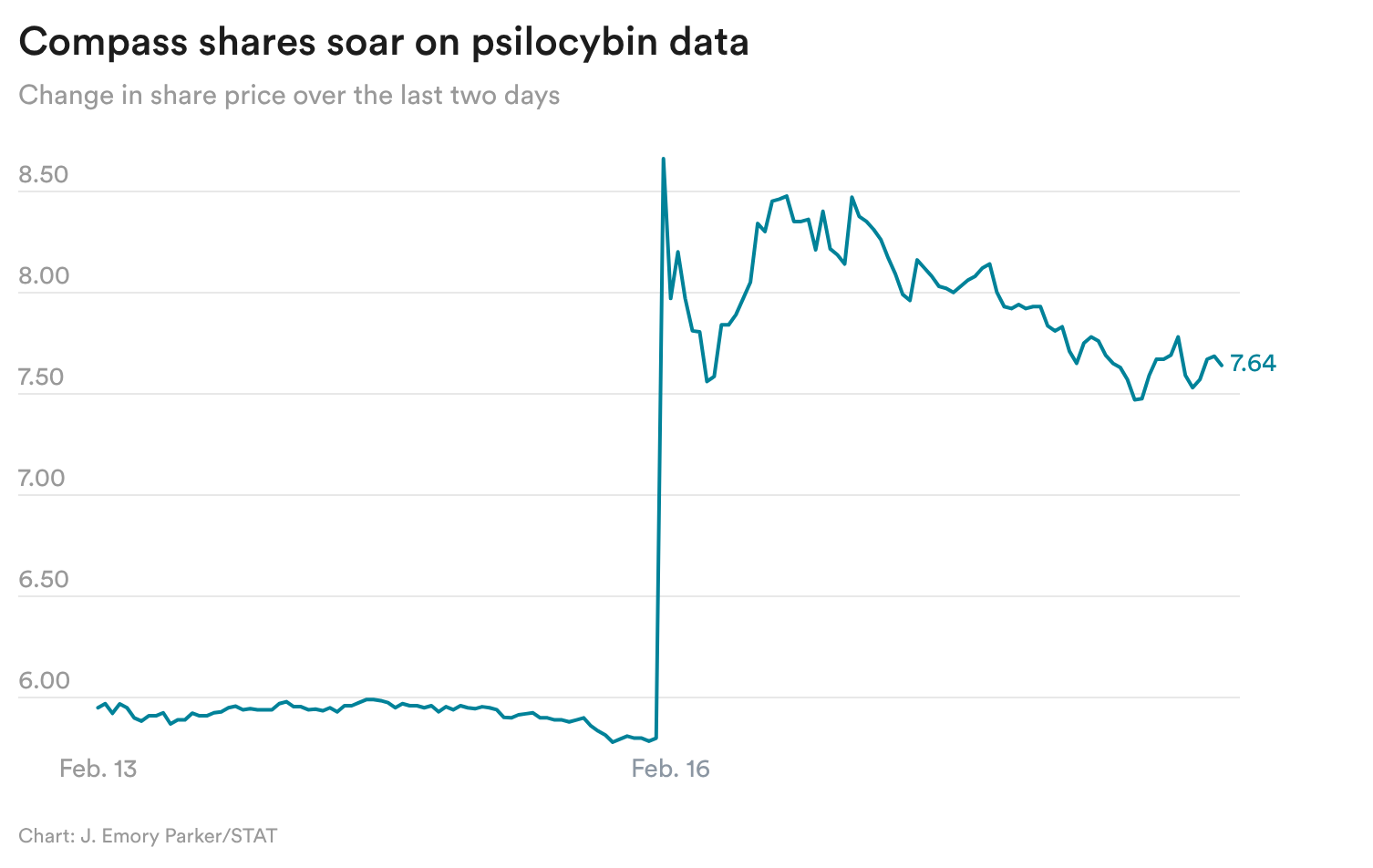
Summary
The FDA has refused to review Moderna's application for its mRNA influenza vaccine, marking a significant setback for the company, which has been a leader in mRNA technology, particularly during the COVID-19 pandemic. This decision raises concerns about the FDA's overall approach to vaccine and drug approvals, as it reflects a trend of placing vaccines under more stringent scrutiny than in previous years. The refusal to review the application has been characterized as a blow to Moderna, which has been at the forefront of vaccine development. Vinay Prasad, a key figure in the FDA's decision-making process, reportedly overruled career FDA scientists in rejecting the application. This action has sparked discussions among pediatricians and other healthcare professionals regarding the agency's stance on vaccines. The implications of the FDA's decision could extend beyond Moderna, potentially affecting the broader landscape of vaccine development and approval processes within the agency. The refusal to review Moderna's flu vaccine application has raised concerns about public trust in vaccine safety and efficacy, particularly as new vaccines are developed and submitted for approval. Stakeholders are closely monitoring the FDA's future actions and policies regarding vaccine approvals, as the agency navigates these challenges. The situation remains dynamic, with ongoing scrutiny of the regulatory processes involved in vaccine approvals.
Advertisement
Cluster Activity
Lindy Score Breakdown (V4.2)
Story Timeline
- 2026-02-10
- 2026-02-11
- 2026-02-12
- 2026-02-13
- 2026-02-17
- 2026-02-18STAT+: Investors cheer Compass’ psilocybin data (current)
Score BreakdownRisk 40
Stories gain Lindy status through source reputation, network consensus, and time survival.
Same Story from 11 sources







Breaking Similar stories





Anti-Lindy Similar stories






